What is the Difference Between Heat and Temperature?
Thermal energy and temperature might sound like the same thing, but it is far from being similar. We will tell you what is the difference between thermal energy and temperature in this article.
Comparison table of the difference between heat and temperature
| Heat | Temperature |
|---|---|
| Heat is one kind of an energy | Temperature is the state of a physical system or a body. It is not energy. |
| When temperature changes, heat is transferred | Temperature changes due to the loss or gain of heat. |
| It can easily transfer from one body to another | It is not exchangeable; it can’t transfer from one body to another |
| The heat present in a body cannot be measured | The temperature of the body can be easily measured. |
| It is measured in Joules | It is measured in Kelvin |
| For example: heat comes out from the stove that warms up the water | For example: As the water warms up, its temperature changes, which can be measured by the thermometer. |
Temperature

It is very important to know about what is the temperature to let you know what is the difference between thermal energy and temperatures.
Temperature is a physical property which means it is measurable. The molecules make up everything in this universe, and the things around you are made up of them. The kinetic energy of these molecules determines the temperature of the body, whether it is cold or hot. Kinetic energy means the molecules are in constant motion, and these create energy.
Temperature holds great significance in the world of natural science. Temperature, for example, is of utmost importance in physics; it tells us about the movement of the particles. In chemistry, it tells us at which temperature a particular reaction will go to completion or which catalyst is essential with respect to the temperature. In geology, the temperature of the earth is very vital to geologists, and like this in other fields, temperature plays an important role. The temperature of the countries is calculated to determine if the country is cool or hot. The top coldest countries are observed by checking their temperature.
How is temperature different from Heat?
Temperature, as said earlier, can be measurable, and it has a SI unit. The temperature has 3 SI units, Kelvin, Celcius, and Fahrenheit.
Always remember that there are two types of the body on the surface, cool and warm. The cool body is always on the lookout for heat, and the warm body wants to get rid of the excess heat. Whenever two bodies come together, the exchange of heat takes place, and it is transferred from the hot body to the cold body till equilibrium is established between them.
Temperature, as stated earlier, has a link with kinetic energy. Kinetic energy, in easy words, is the energy that is created by the movement of the particles in a body. As the particles move with more speed, they produce more heat. It is transferred from one erratic molecule to another calm molecule, and so on. Like the temperature of the body increases, till all molecules are at one state, and when this object or body comes into contact with a cold body. It transfers this excess of energy to the other body, and its molecules are then in erratic motion and increasing the average kinetic energy of the molecules within that body.
Absolute zero temperature, which is -273.16° C, −459.67 °F, or zero Kelvin, is the temperature at which there is no kinetic movement of the molecules, and thus, there are no erratic molecules.
Thermal Energy (Heat Energy)

Likewise, It is very crucial to know about thermal energy to let you know what is the difference between thermal energy and temperatures.
To know about thermal energy, you must be aware of what a system is. A system is a space that is separated from other systems by a clear boundary. There is an energy called, thermal energy that is confined within these boundaries, and this energy is responsible for the temperature of that body or that system. We can also call the body a system. The thermal energy is measured in Joule and Calorie.
We spoke about kinetic energy earlier and how it is responsible for the increase or decrease of temperature here. You learn how thermal energy is the reason the temperature increases and looked at the total kinetic energy of the atoms in a body. It is observed that they are in constant motion, and the constant motion produces energy this energy is known as thermal energy, and it goes from one molecule to another molecule.
How is heat different from temperature?
As the kinetic energy increases, the thermal energy also increases; always remember they are directly proportional to each other. This increase in thermal energy is the reason that the temperature of the body increases.
Always remember that the kinetic energy or the thermal energy that is produced due to the increase in kinetic energy depends upon the object. For example, a cup of water will not have the same kinetic energy as a swimming pool. The average kinetic energy depends on the mass of the body. At the same temperature, e.g., 100 celsius, the kinetic energy of the molecules is the same in both the swimming pool and the cup of water. Still, the swimming pool has more molecules than a cup of water, and thus, the temperature rise in the cup of water will be more in comparison to the swimming pool.
We spoke earlier when we were explaining temperature. You get to know what is the difference between thermal energy and temperatures. What we said was that when two bodies, a body with high temperature, come in contact with a body with low temperature, it means that the heat is transferred from the hot body to the cold body. This is known as a temperature gradient. This heat is basically the thermal energy produced in the body with high kinetic energy. It then gets transferred to the body with low kinetic energy or low temperature.
Always remember that there are three ways via which a body transfers thermal energy, and they are called conduction, convection, and radiation.
Difference between Thermal Energy and Temperatures
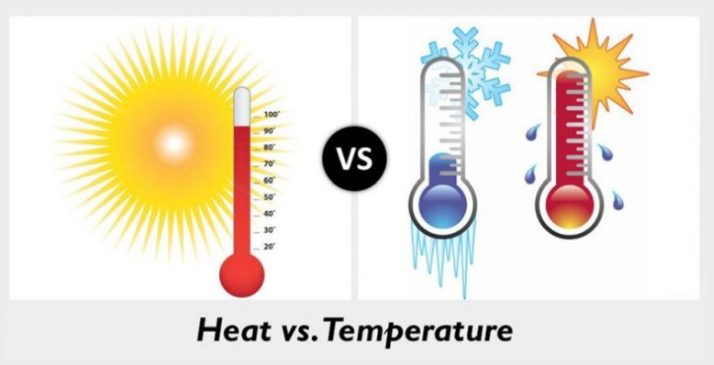
Main difference
Temperature is the average kinetic energy of the molecules in a system. While thermal energy is the sum of the kinetic energy of all the molecules in a system. Take an example of a pen. It is made from multiple molecules. What is the pen? The pen is a system. The pen has 5000 molecules. We take the average of the 5000 molecules to get to know what the average kinetic energy of one molecule is. This is referred to as temperature. The sum of these molecules is known as thermal energy.
Value
Temperature can be negative or positive. If you have seen the temperature on a weather forecast app, you can see that the temperature is positive. It can also go in minus. Thermal energy, on the other hand, is always positive, and it can never be zero. As it is the transfer of heat so we can’t say it can be negative.
Substance
The temperature doesn’t depend on the quantity of the substance. It is the average kinetic energy of the system. Thermal energy depends on the quantity of the substance. It is the total kinetic energy of the system.

-

 Informative3 years ago
Informative3 years ago21 Amazing Fruits That Are Not Round
-

 Science3 years ago
Science3 years agoHow To Make a Dry Ice Bomb at Home? Risks and Precautions
-

 How to3 years ago
How to3 years agoHow to Put a Tampon On: Step by Step Guide
-

 How to3 years ago
How to3 years agoHere’s How to Know When The Oil Cartridge Is Empty
-
 Informative3 years ago
Informative3 years agoElf Ear Surgery: Cost, Procedure, and Risks
-
 How to3 years ago
How to3 years agoFixed: The Torrent You Are Trying To Add is Already in The List
-

 How to3 years ago
How to3 years agoHow To Thaw Frozen Pipes Underground
-

 How to3 years ago
How to3 years agoSolved: How to Change Your Age on TikTok? (2021)