Explained: How Many Valence Electrons Does Carbon Have

It is one of the common questions in basic chemistry – its importance increases, and it is studied in more depth as your interest in Chemistry increases, or you take it as a major. The answer to the question that How many valance electrons does carbon have? is four.
The Electronic Configuration of Carbon
There are many energy levels around the nucleus of an atom and they are attracted by a strong electrostatic force towards the center where the nucleus is situated. The number of electrons in these energy levels and their arrangement is known as electronic configuration.

Carbon’s electronic configuration
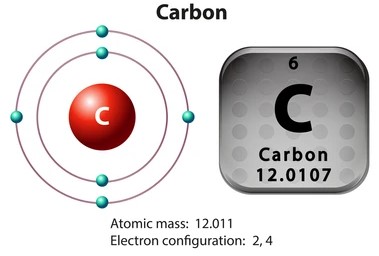
The energy levels in carbon are two and if you look at the diagram above the 1s2 represents the first energy level while the 2s2 and 2p2 represent the second energy level. The first energy level contains 2 electrons and the second energy level has 4 electrons. This can also be represented by something like this. This answers the question How many valence electrons does carbon have really well.
Carbon
The above diagram shows the energy levels in a much simplified manner. The first energy level, also referred to as the 1s, can only accommodate 2 electrons, and in the case of carbon, it has been fully occupied. The second energy level can accommodate a total of 8 electrons, but carbon hasn’t fully occupied it and only has 4 electrons in it.
The above diagram also has a snippet from the periodic table of the element carbon, and it shows the proton number (6); one can easily derive the total number of electrons in an atom from that.
There is another method of knowing the electrons in the last shell of carbon and that is by looking at the periodic table. Carbon is in the second row of the periodic table and as you go from lithium with a 1 one electron in its last shell, and as you go from left to right on the periodic table add one more electron in the outermost shell of the next element, and as you reach carbon the valence electrons are 4.
Similar Reads: Explained: How Many Valence Electrons Does Calcium Have?
Noble Gas Configuration And Carbon?
The valency of carbon is 4 as stated earlier carbon in the second energy level can still have room for a total of 8 electrons but it has only 4 electrons. By attaining the other 4 electrons it will then satisfy the octet rule and its valence shell electrons will be like a noble gas, the most stable elements on the periodic table and the aim of every element is to go and attain this electronic configuration.
How many valence electrons does carbon have?’ was the main focus but the answer to it is 4 is a mystery that will be answered below.
How Does Carbon Attain Noble Gas Configuration?
Nature has made carbon such that it has 4 valence electrons making it attain certain properties that make it unstable, but it also makes stability for it very easy through sharing.
Noble gas configuration can be attained through an ionic reaction between a metal, and a non-metal, or it can be attained through the process of covalent bond formation. Whichever way it is, the main aim is to fill the energy level in which the valence electrons lie and attain the stability of the noble gas.
Always remember that Carbon is a tetravalent in nature, which means the ionic bond formation is out of the question, and it can not lose the 4 valence electrons but share it with another element or another carbon. In ionic bonds, you can either gain or lose electrons from the outer shell to obtain the noble gas configuration but in the case of a covalent bond, the sharing of electrons occurs between the outer shells of two or more elements.
Step by step guide
- The property of Carbon is to gain electrons through sharing and can’t lose them or gain them via ionic bonding. It has 4 valence electrons in its outer shell, making it a tetravalent element.
- The noble gas configuration that it gains is through the octet rule.
- It is tetravalent in nature, so either it will share electrons with another non-metal or with a carbon element in itself.
- That way it will gain four more electrons and become stable while forming a molecule in the process.
Carbon After Attaining The Noble Gas Configuration
After attaining the noble gas configuration, the carbon atom will now have 8 electrons in the outer shell, and it will now be completed. An example of this is below.
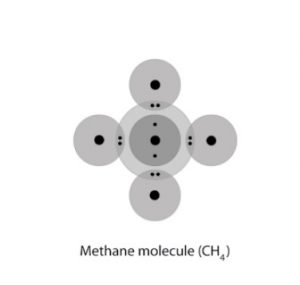
CH4 Covalent Bonding
After this, the electronic configuration of carbon would be 1s2 2s2 2p6 and will be more stable and not erratic like a single carbon atom.
Valence Electrons And Carbon Mostly Asked Questions
You need to clearly define what we mean by valance electrons and how many do carbon have.
What is the valance electron?
It is the electron in the outermost shell of an element.
How many total electrons in Carbon?
There are 6 electrons in carbon.
How many shells does Carbon have?
Carbon has two shells
How many electrons in the first shell of carbon?
It has 2 electrons
How many electrons in the second shell of carbon?
It has 4 electrons.
What is valency?
It is the total number of electrons an atom can lose, gain or share at time to get the stable gas configuration of a noble gas.

-

 Informative3 years ago
Informative3 years ago21 Amazing Fruits That Are Not Round
-

 Science3 years ago
Science3 years agoHow To Make a Dry Ice Bomb at Home? Risks and Precautions
-

 How to3 years ago
How to3 years agoHow to Put a Tampon On: Step by Step Guide
-

 How to3 years ago
How to3 years agoHere’s How to Know When The Oil Cartridge Is Empty
-
 Informative3 years ago
Informative3 years agoElf Ear Surgery: Cost, Procedure, and Risks
-
 How to3 years ago
How to3 years agoFixed: The Torrent You Are Trying To Add is Already in The List
-

 How to3 years ago
How to3 years agoHow To Thaw Frozen Pipes Underground
-

 How to3 years ago
How to3 years agoSolved: How to Change Your Age on TikTok? (2021)